Cleanrooms GMP IFS
Cleanrooms GMP IFS are ISO classified areas in the medical sector and food industry with a strict quality assurance. Limiting germination and contamination is the critical success factor. HIGHCARE delivers metal air-conditioned cleanrooms with a unique air ventilation technology for a complete flushing of the area, including a UVC unit in the air treatment for protection against harmful micro-organisms such as viruses and moulds. Thanks to the modular system, the construction time is short.
GMP EMA
HIGHCARE-cleanrooms worden gebruikt voor onderzoek, productie en of verpakkingsruimte. GMP EMA Grade A t/m HIGHCARE cleanrooms are used for research and production as well as packaging areas. GMP EMA Grade A to D are the standard quality assurances for the human, veterinary, pharmaceutical and cosmetic industries. In the food industry and hospitality sector, quality requirements such as those laid down in ISO 22000, IFS, BRC and HACCP, are applied. HIGHCARE builds internationally certified cleanrooms; including air treatment, ISO certificate and delivered ready for use.
<ISO 14644>
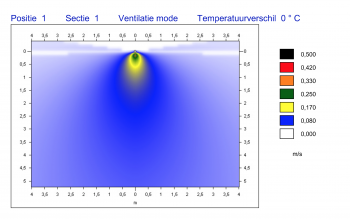
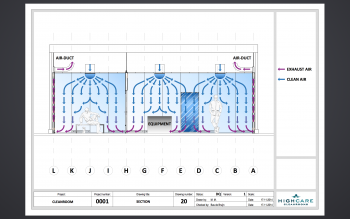
AIRFLOW TECHNOLOGY
The ingenious airflow is one of the characteristics of a HIGHCARE cleanroom. Using a unique technology, purified air is blown in via the ceiling and extracted at skirting board level. A GMP cleanroom should be flush and sanitary and “washes” a space with air, so to speak. The metal cleanrooms are easy to clean. A sound technical installation guarantees a continuous flow of filtered air. You determine the necessary standard and classification. Commonly used classifications are ISO 6 or lower.
BOX-IN-BOX AND STAND-ALONE
A Highcare cleanroom box-in-box system is stand-alone and is built under a freestanding moveable intermediate floor, on which the technical installation will be placed. Contaminating equipment is kept outside the cleanroom. This easy to (dis)assemble box-in-box system makes it possible to carry out repairs or adjustments locally without bringing the production process to a complete standstill. Modifications are easy to carry out thanks to the simple click-and-click system, possibly by your own staff and therefore at a lower cost.
GMP CLEANROOM STRICT QUALITY ASSURANCE
GMP Cleanrooms have a strict quality assurance. The system therefore consists of durable materials. The complete cleanroom, including the intermediate floor, can be disassembled, moved and easily reassembled. HIGHCARE builds according to the latest technology, the IFD system.
<IFD Building Technology>
HIGHCARE Cleanrooms ensures a punctual realisation in the shortest possible construction period.